Research Type: Reports
We conduct thorough investigations and studies of related various aspects of drug prices. When concluded, they are published to share our findings with academic, policymakers, and the public.
-
Modeling P-quad
-
Competitive Acquisition Program
-
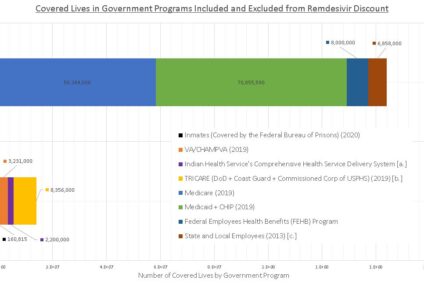
Remdesivir Less Expensive for “Government Programs”? Not So Fast.
-

Blueprints for Indication-Specific Pricing
-
Fact Check: Time to Market for New Drugs in U.S. Versus Other Countries
-

Impact of President’s Budget and Point of Sale Rebate Proposal for Part D: 10 Year Projection
-

Overview of Original Subscription Model and Features of LA and WA Proposals
-
DPL Responds to HHS RFI for IPI Drug Pricing Model (Nov. 30, 2018)
-
DPL responds to HHS Blueprint (May 16, 2018)
-
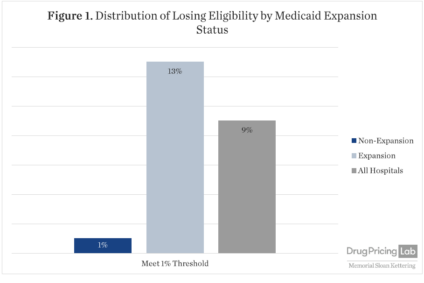
Impact of President Trump’s Proposed 340B Hospital Eligibility Threshold