The New York Times ran a ‘Fact Check’ about the recent Trump Administration proposal to pilot alternative reimbursement for Part B drugs. But, in an effort to clear up potential confusion, the article made its own errors. Here are a few.
1.The ‘fact check’ took issue with the word “voluntary” in Secretary Azar’s assertion that “U.S. drug companies voluntarily cut the price of drugs overseas…,” pointing out that companies lower prices to meet price ceilings countries set based on a variety of means, including assessing the value of the products, and checking against what other countries pay.
- Problem: While the word ‘voluntarily’ might imply that companies lower their prices cheerfully, if we are talking facts, the opposite of ‘voluntarily’ is not ‘grudgingly’ but ‘involuntarily.’ To be clear, the pharmaceutical market in the US and in other OECD countries is, in fact, a voluntary one – countries (and payers generally) arrive at prices they are willing to pay; drug companies decide, voluntarily, to sell their products at those prices, or not.
- The fact checker could have taken issue with the phrase “U.S. drug companies” as it implies that the pharmaceutical industry is a U.S. based one. This is incorrect, five out of ten of the largest biopharma companies in the world are based outside the U.S.
2. The ‘fact check’ took issue with the statement that “In Medicare Part B today, the government gets the bill, and we just blindly pay for it…there is no negotiation,” suggesting that in fact the government reimbursement for Part B drugs piggybacks off of discounts achieved through negotiation in U.S. commercial markets.
- Problem: The fact-checker lists several entities that do not actually negotiate over prices of Part B drugs. Pharmacy Benefit Managers (PBMs) play essentially no role in negotiating for Part B (medical benefit) drugs. It also lists health insurers, but in nearly all cases they negotiate reimbursement rates with physicians and hospitals for the use of medical benefit drugs, not with drug manufacturers over the price of those drugs. This system is called “buy and bill” and is outlined here.
- Problem: The fact-checker says that price concessions on Part B drugs are treated as trade secrets. This is incorrect, at least in the aggregate. The net prices (called the Average Sales Prices) for Part B drugs is published in the aggregate every quarter by CMS, these prices form the basis of Medicare’s reimbursement. Here are the tables for the most recent quarter. Some sales of Part B drugs are discounted by law, such as sales to 340B hospitals. These are excluded from ASP calculations.
- Problem: The fact-checker incorrectly implies that the concessions achieved by these various negotiations achieves discounts well below list price for Part B drug, citing a range of -15% to -35%. The list price is a colloquial term that generally refers to the Wholesale Acquisition Cost (the WAC) of a Part B drug. The Medicare Payment Advisory Committee in 2017 examined the difference between WAC and ASP for eight drug launches in among the top 20 highest expenditure Part B drugs in 2014, and none of the eight drugs were discounted more than 3%.
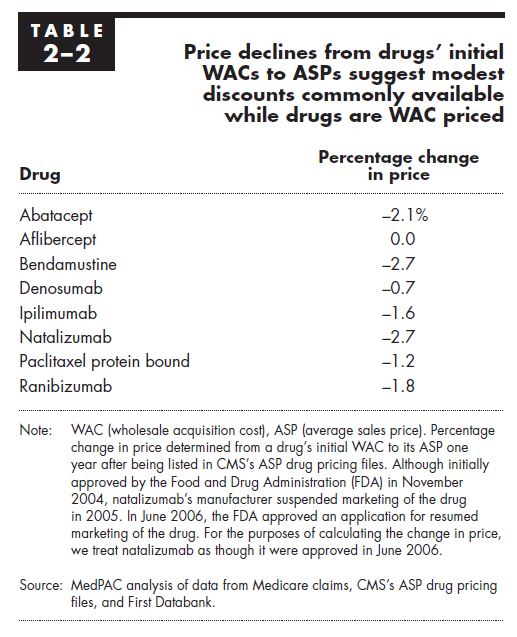
Source: MedPAC, Report to the Congress: Medicare and the Health Care Delivery System
3. The Fact Checker quotes the well-respected oncologist Debra Patt, MD, who raises concerns about the administration’s proposed changes. But it lists her affiliation as working at Texas Oncology, a physician practice. It fails to note that per Dr. Patt’s LinkedIn profile, she actually also works for both the drug wholesaler McKesson and for its subsidiary US Oncology that has an affiliation with Texas Oncology. Her profile reads “Dr. Patt serves as a medical director McKesson Specialty Health and The US Oncology Network,” and lists this affiliation as commencing in 2016. [A previous version listed Texas Oncology as owned by US Oncology.]